
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (6): 531-540.DOI: 10.12300/j.issn.1674-5817.2022.014
• Animal Models of Human Diseases • Previous Articles Next Articles
Liya YANG1, Tao SONG2, Jialin HE3, Yiming GUO3, Mingkang QI3, Hanbi WANG3( )(
)( ), Huiping WANG1(
), Huiping WANG1( )(
)( )
)
Received:2022-02-11
Revised:2022-07-26
Online:2022-12-25
Published:2022-12-25
Contact:
Hanbi WANG, Huiping WANG
CLC Number:
Liya YANG,Tao SONG,Jialin HE,et al. Establishment of a Vaginal Atrophy Rat Model and its Application in Pharmacodynamic Evaluation[J]. Laboratory Animal and Comparative Medicine, 2022, 42(6): 531-540. DOI: 10.12300/j.issn.1674-5817.2022.014.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.014
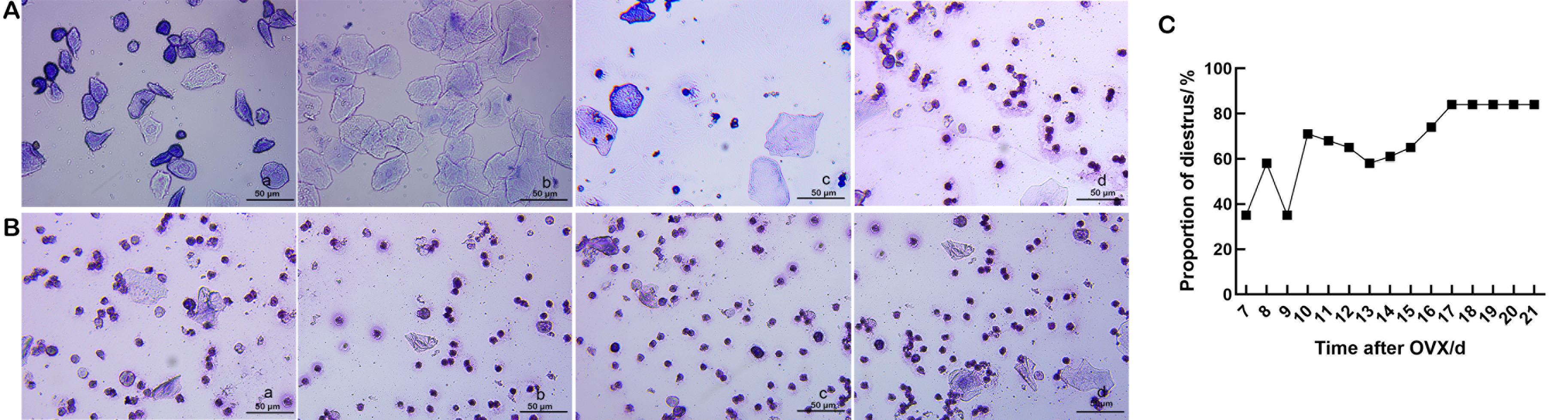
Figure 1 Vaginal smears after ovariectomy (OVX) of rats in each group (Wright's staining, ×40) (A and B)and the proportion of the diestrus period (C)Note: A, regular changes in the estrous cycle were observed in the vaginal smears of the normal group (a–d show pre-estrous, estrous, post-estrous, and diestrus periods, respectively). B, vaginal smears after OVX in the model establishment group (a–d was 18, 19, 20, and 21 days after OVX, respectively, showing the diestrus period). C, the proportion of estrous/diestrus on days 7–21 after OVX in the model establishment group (21 rats), model group, drug group 1, drug group 2 and solvent control group (10 rats in each group) (n=61). Scale bars in all panels are 50 μm.

Figure 2 Body weight (A) and uterine wet weight/body weight (B) of rats from day 15–21 after ovariectomy (OVX)Note: A, weight changes of rats in the normal control and model establishment groups during days 15–21; B, changes in uterus wet weight/body weight at days 15–21 after OVX in the model establishment group. Daily number of rats n=3. *P<0.05, compared with the model establishment group.
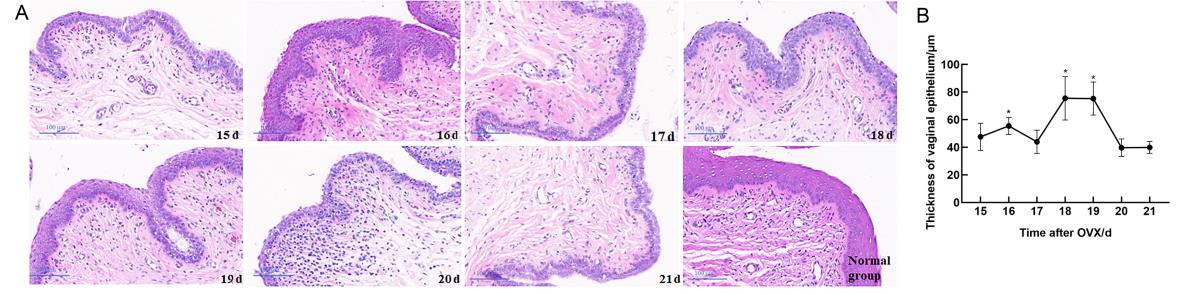
Figure 3 Histopathological changes of vaginal in rats after ovariectomy (OVX) and the thickness of vaginal epitheliumNote:A, HE staining of vaginal tissues of rats in the model establishment and normal groups days 15–21 after OVX (scale bar is 100 μm); B, the thickness of the vaginal epithelium in the model establishment group days 15–21 after OVX (n=3, compared with 21 days after OVX, *P<0.05).
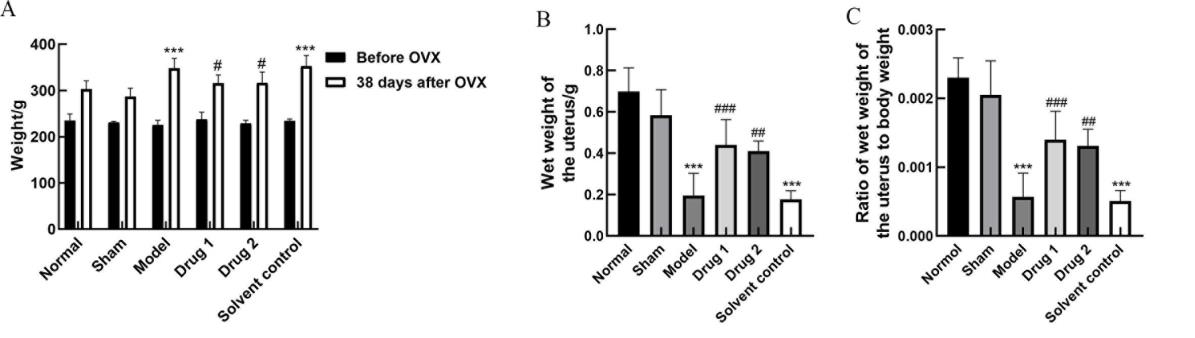
Figure 4 Body weight (A), uterine wet weight (B), and uterine wet weight/ body weight (C) of rats in each group after drug treatmentNote:Drug group 1, drug group 2, and solvent control group were treated with promestriene, Colpotrofin?, and solvent control for 14 days, respectively.In each group, n=10. ***P<0.001, compared with the sham group; #P<0.05, ##P<0.01, and ###P<0.001, compared with the model group.
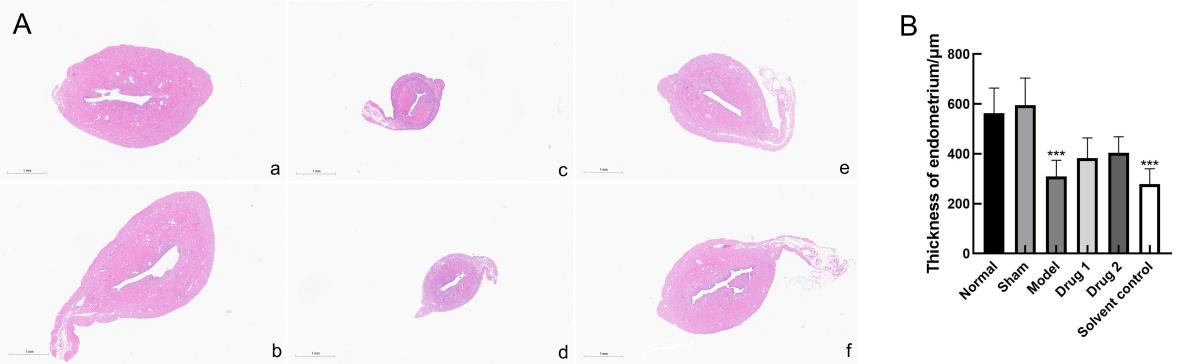
Figure 5 Histopathological changes of uterus tissue(HE, ×2)(A) and thickness of the endometrium (B)Note:a, normal group; b, sham group; c, model group; d, solvent control group; e, drug group 1(promestriene); f, drug group 2 (Colpotrofin?). The scale bar of the panel is 1 mm. In each group, n=10. ***P<0.001, compared with the sham group.
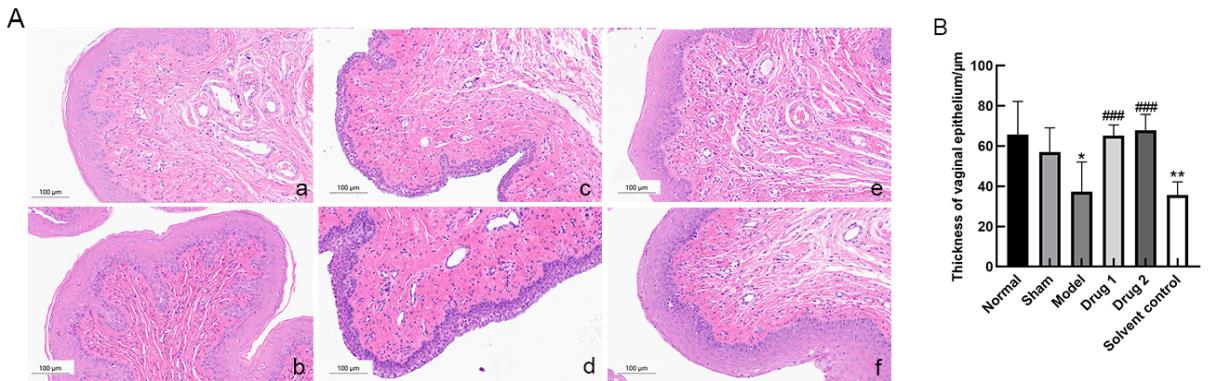
Figure 6 Histopathological changes of vaginal tissue (HE, ×20)(A) and thickness of the vaginal epithelium (B)Note:a, normal group; b, sham group; c, model group; d, solvent control group; e, drug group 1(promestriene); f, drug group 2 (Colpotrofin?). The scale bar of the panel is 100 μm. *P<0.05, and **P<0.01, compared with the sham group; ###P<0.001, compared with the model group.
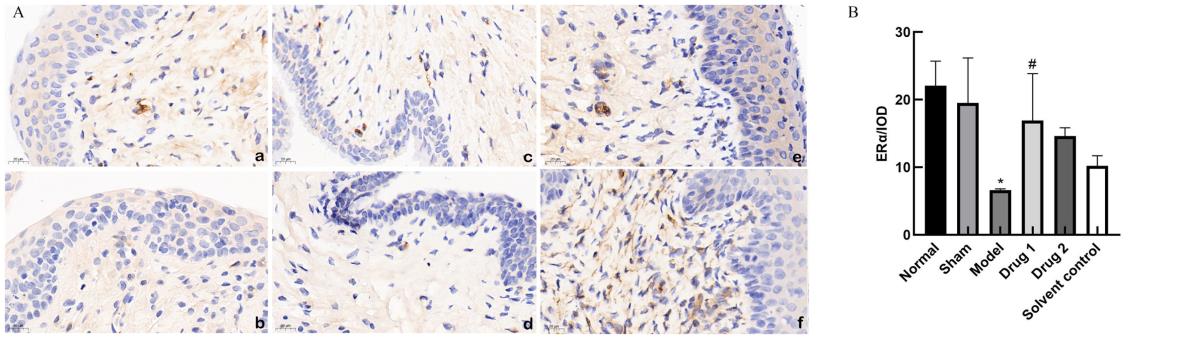
Figure 7 Immunohistochemistry (DAB, ×20)(A) and integral optical density (B) of ERα protein expression in rat vaginaNote:ERα, estrogen receptors-α. a, normal group; b, sham group; c, model group; d, solvent control group; e, drug group 1(promestriene); f, drug group 2 (Colpotrofin?). The scale bar of the panel is 20 μm. *P<0.05, compared with the sham group; #P<0.05, compared with the model group.
| 1 | NAPPI R E, MARTINI E, CUCINELLA L, et al. Addressing vulvovaginal atrophy (VVA)/genitourinary syndrome of menopause (GSM) for healthy aging in women[J]. Front Endocrinol (Lausanne), 2019, 10:561. DOI:10.3389/fendo. 2019. 00561 . |
| 2 | MALDONADO P A, MONTOYA T I, ACEVEDO J F, et al. Effects of vaginal conjugated equine estrogens and ospemifene on the rat vaginal wall and lower urinary tract[J]. Biol Reprod, 2016, 96(1):81-92. DOI:10.1095/biolreprod.116. 144428 . |
| 3 | YOU S, LIU S B, DONG X J, et al. Intravaginal administration of human type III collagen-derived biomaterial with high cell-adhesion activity to treat vaginal atrophy in rats[J]. ACS Biomater Sci Eng, 2020, 6(4):1977-1988. DOI:10.1021/acsbiomaterials.9b01649 . |
| 4 | ZANNI P C, NEGRI M, SALCI T P, et al. Animal models for the effective development of atrophic vaginitis therapies: possibilities and limitations[J]. Expert Opin Drug Discov, 2014,9(3):269-281. DOI:10.1517/17460441.2014.877883 . |
| 5 | 吴琼. 雌激素联合甲硝唑治疗萎缩性阴道炎的临床疗效及其安全性[J]. 临床合理用药杂志, 2021, 14(21):145-147. DOI:10.15887/j.cnki.13-1389/r.2021.21.053 . |
| WU Q. Clinical efficacy and safety of estrogen combined with metronidazole in the treatment of atrophic vaginitis[J]. Chin J Clin Ration Drug Use, 2021, 14(21):145-147. DOI:10.15887/j.cnki.13-1389/r.2021.21.053 . | |
| 6 | DONDERS G G G, RUBAN K, BELLEN G,et al. Pharmaco-therapy for the treatment of vaginal atrophy[J]. Expert Opin Pharmacother, 2019, 20(7):821-835. DOI:10.1080/14656566. 2019. 1574752 . |
| 7 | MAYER L P, DEVINE P J, DYER C A, et al. The follicle-deplete mouse ovary produces androgen[J]. Biol Reprod, 2004, 71(1):130-138. DOI:10.1095/biolreprod.103.016113 . |
| 8 | ACOSTA J I, MAYER L, TALBOOM J S, et al. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system[J]. Endocrinology, 2009, 150(9):4248-4259. DOI:10.1210/en.2008-1802 . |
| 9 | SOCIETY N A M. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society[J]. Menopause, 2007, 14(3):370-371. DOI:10.1097/gme.0b013e3180533b2a . |
| 10 | LÓPEZ-BELMONTE J, NIETO C, ESTEVEZ J, et al. Comparative uterine effects on ovariectomized rats after repeated treatment with different vaginal estrogen formulations[J]. Maturitas, 2012, 72(4):353-358. DOI:10.1016/j.maturitas.2012.05.007 . |
| 11 | PUNNONEN R, VILSKA S, GRÖNROOS M, et al. The vaginal absorption of oestrogens in post-menopausal women[J]. Maturitas, 1980, 2(4):321-326. DOI:10.1016/0378-5122(80)90034-1 . |
| 12 | CECCARELLI S, D'AMICI S, VESCARELLI E, et al. Topical KGF treatment as a therapeutic strategy for vaginal atrophy in a model of ovariectomized mice[J]. J Cell Mol Med, 2014, 18(9):1895-1907. DOI:10.1111/jcmm.12334 . |
| 13 | VAILATI S, MELLONI E, RISCASSI E, et al. Evaluation of the effects of a new intravaginal gel, containing purified bovine colostrum, on vaginal blood flow and vaginal atrophy in ovariectomized rat[J]. Sex Med, 2013, 1(2):35-43. DOI:10.1002/sm2.8 . |
| 14 | WOLFF J P, CACHELOU R, GUÉRITÉE N. Absence of systemic hormonal effects in an oestradiol diether topically active on the vaginal mucosa[J]. Maturitas, 1982, 4(4):239-246. DOI:10.1016/0378-5122(82)90054-8 . |
| 15 | PUP L D. Management of vaginal dryness and dyspareunia in estrogen sensitive cancer patients[J]. Gynecol Endocrinol, 2012, 28(9):740-745. DOI:10.3109/09513590.2011.652717 . |
| 16 | 闫丽, 温和, 唐桂毅, 等. 大鼠阴道细胞涂片不同染色方法在动情周期判定中的价值[J]. 药物评价研究, 2020, 43(1):72-76. DOI:10.7501/j.issn.1674-6376.2020.01.012 . |
| YAN L, WEN H, TANG G Y, et al. Diagnostic value of three staining methods for rat vaginal cell smears in determination of estrous cycle[J]. Drug Eval Res, 2020, 43(1):72-76. DOI:10.7501/j.issn.1674-6376.2020.01.012 . | |
| 17 | 刘翠萍, 张颖, 李宁莉. 干扰素联合红外线局部照射治疗人乳头瘤病毒及相关阴道炎和宫颈炎大鼠的作用及相关机制研究[J]. 临床和实验医学杂志, 2021, 20(13):1353-1357. DOI:10.3969/j.issn.1671-4695.2021.13.003 . |
| LIU C P, ZHANG Y, LI N L. Effect and mechanism of interferon combined with infrared local radiation in the treatment of rats with HPV related vaginitis and cervicitis[J]. J Clin Exp Med, 2021, 20(13):1353-1357. DOI:10.3969/j.issn.1671-4695. 2021. 13.003 . | |
| 18 | LI T, MA Y Y, ZHANG H, et al. Differential regulation of morphology and estrogen receptor-alpha expression in the vagina of ovariectomized adult virgin rats by estrogen replacement: a histological study[J]. Int J Endocrinol, 2016, 2016:1093512. DOI:10.1155/2016/1093512 . |
| 19 | 游爽. Ⅲ型胶原蛋白对大鼠阴道萎缩的作用及机制研究[D]. 重庆: 重庆医科大学, 2020. |
| YOU S. The effect and mechanism of type Ⅲ collagen on vaginal atrophy in rats [D].Chongqing: Chongqing Medical University, 2020. | |
| 20 | 李淑桢, 王琦, 李沁园, 等. 基于下丘脑-垂体-卵巢轴探讨藏药二十五味鬼臼丸对绝经后骨质疏松症大鼠的干预作用[J]. 中草药, 2021, 52(20):6282-6290. DOI:10.7501/j.issn.0253-2670.2021.20.019 . |
| LI S Z, WANG Q, LI Q Y, et al. Study on intervention effect of Tibetan medicine Ershiwuwei Guijiu Pill on PMOP rats based on HPOA[J]. Chin Tradit Herb Drugs, 2021, 52(20):6282-6290. DOI:10.7501/j.issn.0253-2670.2021.20.019 . | |
| 21 | YANG Q N, WANG C Y, JIN Y, et al. Disocin prevents postmenopausal atherosclerosis in ovariectomized LDLR-/- mice through a PGC-1α/ERα pathway leading to promotion of autophagy and inhibition of oxidative stress, inflammation and apoptosis[J]. Pharmacol Res, 2019, 148:104414. DOI:10.1016/j.phrs.2019.104414 . |
| 22 | 谢冰颖, 黄景文, 陈赛楠, 等. 补肾法对围绝经期综合征大鼠性激素及卵巢雌激素受体的影响[J]. 福建中医药, 2021, 52(10):11-13, 22. DOI:10.13260/j.cnki.jfjtcm.012341 . |
| XIE B Y, HUANG J W, CHEN S N, et al. Effect of kidney-invigorating method on the levels of sex hormone and ovarian estrogen receptor in perimenopausal syndrome rats[J]. Fujian J Tradit Chin Med, 2021, 52(10):11-13, 22. DOI:10.13260/j.cnki.jfjtcm.012341 . |
| [1] | LIU Liyu, JI Bo, LIU Xiaoxuan, FANG Yang, ZHANG Ling, GUO Tingting, QUAN Ye, LI Hewen, LIU Yitian. Exploration of Rat Fetal Lung Tissue Fixation Methods [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 432-438. |
| [2] | LIU Ziqi, LI Yunying, LI Qin, LI Yuanhan, HE Fangyan, WEN Weibo. Research Progress on Animal Models of Gastric Ulcer of Spleen-Stomach Deficiency Cold Type [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-12. |
| [3] | TAN Dengxu, MA Yifan, LIU Ke, ZHANG Yanying, SHI Changhong. Reshaping Intercellular Interactions: Empowering the Exploration of Disease Mechanisms and Therapies Using Organoid Co-Culture Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 309-317. |
| [4] | SHEN Huangyi, HUANG Yufei, YANG Yunpeng. Research Progress on Characteristics Analysis of Gut Microbiota and Its Sex Differences in Laboratory Animals [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 349-359. |
| [5] | TONG Xiyang, QUE Changtian, ZHANG Feng, ZHAO Lu, WANG Hongping. Analysis of Common Causes of Out-of-Specification Results in the Test for Depressor Substances [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 331-339. |
| [6] | LUO Lianlian, YUAN Yanchun, WANG Junling, SHI Guangsen. Advances in Mouse Models of Amyotrophic Lateral Sclerosis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 290-299. |
| [7] | XIAO Linlin, YANG Yixuan, LI Shanshan, LUO Lanshiyu, YIN Siwei, SUN Juming, SHI Wei, OUYANG Yiqiang, LI Xiyi. Establishment of a Rat Model of Alzheimer's Disease by Introducing Human Triple Mutant APP Gene into Hippocampus via Brain Stereotactic Technology [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 269-278. |
| [8] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [9] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [10] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [11] | XIAO Wenxian, LÜ Longbao. Research Progress on Human Ovarian Aging Using Non-Human Primates as Laboratory Animals [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 47-54. |
| [12] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [13] | LIU Yishu, CAI Liping. Advances and Challenges of Using Experimental Pigs in Da Vinci Surgical Robot Training [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 667-674. |
| [14] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| [15] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||