
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (2): 162-179.DOI: 10.12300/j.issn.1674-5817.2023.159
• Animal Models of Human Diseases • Previous Articles Next Articles
Ruiqi LI( ), Han DUAN(
), Han DUAN( ), Luo GAN, Yuan ZHENG(
), Luo GAN, Yuan ZHENG( )(
)( ), Wen YANG(
), Wen YANG( )(
)( )
)
Received:2023-11-13
Revised:2024-02-09
Online:2024-04-25
Published:2024-04-25
Contact:
Yuan ZHENG, Wen YANG
CLC Number:
Ruiqi LI,Han DUAN,Luo GAN,et al. Advantages of Ciona intestinalis as a Model Organism and Its Applications[J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 162-179. DOI: 10.12300/j.issn.1674-5817.2023.159.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.159
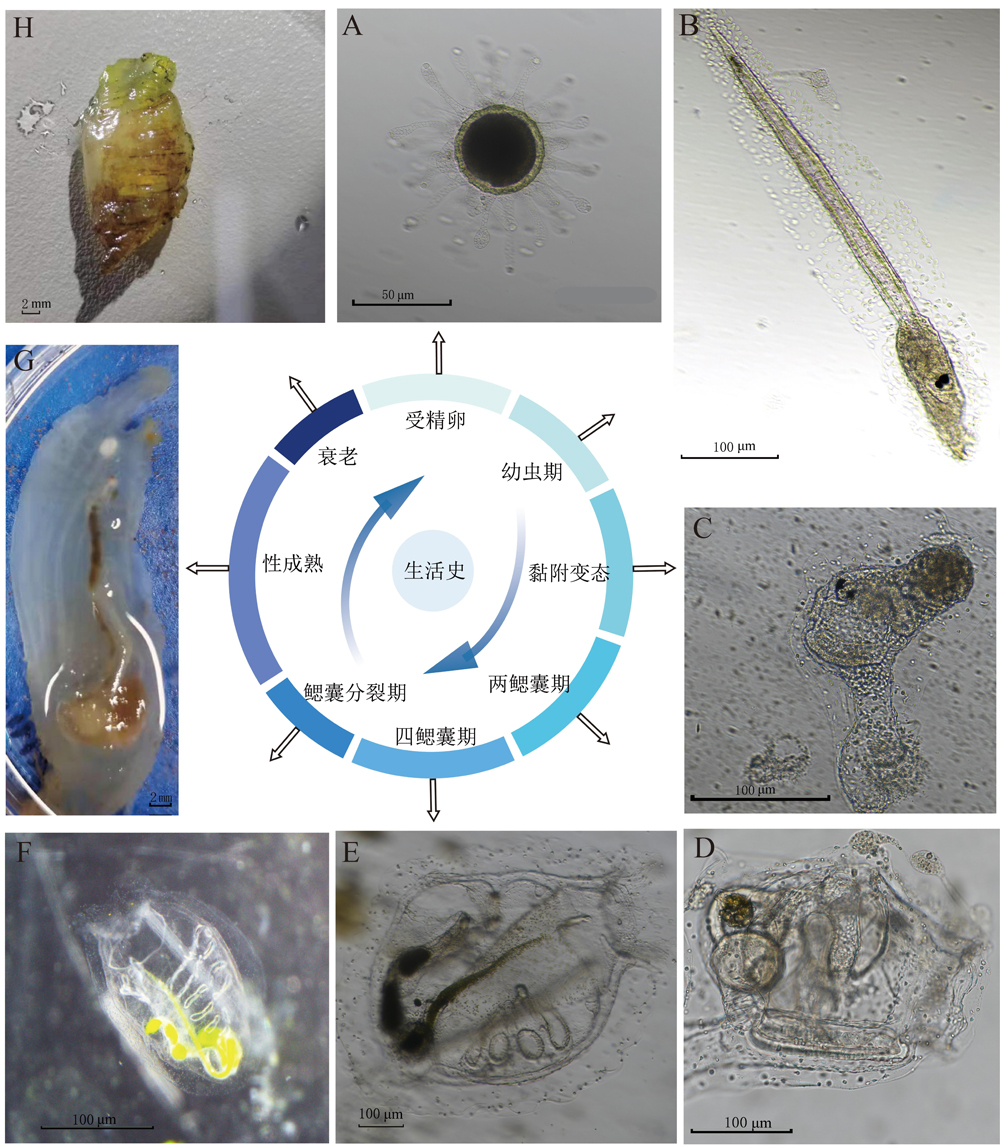
Figure 2 Life history of Ciona intestinalisNote:A, Fertilized egg (scale bar: 50 μm); B, Attached larva (scale bar: 100 μm); C, Juvenile during metamorphosis (scale bar: 100 μm); D, Two-branchial sac stage larva (scale bar: 100 μm); E, Four-branchial sac stage larva (scale bar: 100 μm); F, Juvenile during branchial sac division (scale bar: 100 μm); G, Sexually mature adult (scale bar: 2 mm); H, Aged adult (scale bar: 2 mm).
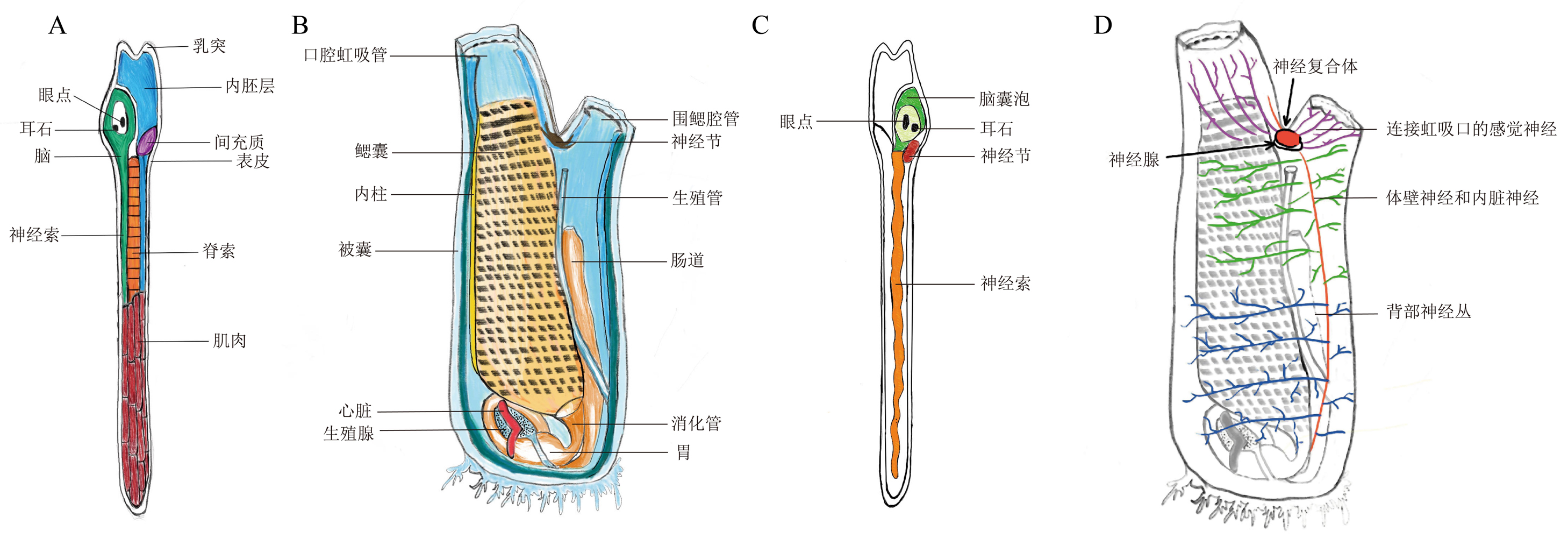
Figure 3 Structural diagrams and central nervous system diagrams of Ciona intestinalis larva and adultNote:A, Schematic diagram of larval structure depicting six tissues: the endoderm (blue), mesenchyma (purple), central nervous system (green), notochord (orange), muscle (reddish-brown), and the outermost layer of epidermis; B, Schematic diagram of the adult's internal structure, showing the central nervous system consisting of sensory vesicles (the brain, which includes sensory organs such as ocellus and otolith), nerve cords, and ganglia; C, Schematic diagram of the larval central nervous system; D, Schematic diagram of the adult central nervous system.
资源名称 Resource names | 网址 Website | 类型 Type | 主要内容 Main content |
|---|---|---|---|
| Joint genome institute | https://genome.jgi.doe.gov/portal/ | 数据库 | 基因组 |
| Ghost Database | http://ghost.zool.kyoto-u.ac.jp/cgi-bin/gb2/gbrowse/kh | 数据库(日语) | 基因组和cDNA |
| Aniseed genome browser | http://www.aniseed.cnrs.fr/ | 数据库 | 基因组 |
| Smith research lab | http://labs.mcdb.ucsb.edu/smith/william | 公众号 | 神经系统发育和功能 |
| UniProt | https://www.uniprot.org/ | 数据库 | 蛋白质组 |
| Ensemble | http://asia.ensemble.org | 数据库 | 基因组 |
| NCBI | http://www.ncbi.nlm.nih.gov/dbEST/dbEST_summary.html | 数据库 | 基因的时空表达信息 |
| NBRP | http://nbrp.jp/ | 数据库(日语) | cDNA |
Database of Tunicate Gene Regulation | http://dbtgr.hgc.jp/ | 数据库 | 组织特异性基因5'侧翼的顺式调控元件数据库 |
| NCBI | http://www.ncbi.nlm.nih.gov/geo/ | 数据库 | 基因表达谱 |
| Tefor | http://crispor.tefor.vet | 数据库 | CRISPOR引物在线设计 |
| PrimerX | http://www.bioinformatics.org/primerx/cgi-bin/DNA_1.cgi | 数据库 | 基因突变引物在线设计 |
| EDomics | http://edomics.qnlm.ac/#/home | 数据库 | 基因组和转录组等 |
Table 1 Genome-related databases of Ciona intestinalis
资源名称 Resource names | 网址 Website | 类型 Type | 主要内容 Main content |
|---|---|---|---|
| Joint genome institute | https://genome.jgi.doe.gov/portal/ | 数据库 | 基因组 |
| Ghost Database | http://ghost.zool.kyoto-u.ac.jp/cgi-bin/gb2/gbrowse/kh | 数据库(日语) | 基因组和cDNA |
| Aniseed genome browser | http://www.aniseed.cnrs.fr/ | 数据库 | 基因组 |
| Smith research lab | http://labs.mcdb.ucsb.edu/smith/william | 公众号 | 神经系统发育和功能 |
| UniProt | https://www.uniprot.org/ | 数据库 | 蛋白质组 |
| Ensemble | http://asia.ensemble.org | 数据库 | 基因组 |
| NCBI | http://www.ncbi.nlm.nih.gov/dbEST/dbEST_summary.html | 数据库 | 基因的时空表达信息 |
| NBRP | http://nbrp.jp/ | 数据库(日语) | cDNA |
Database of Tunicate Gene Regulation | http://dbtgr.hgc.jp/ | 数据库 | 组织特异性基因5'侧翼的顺式调控元件数据库 |
| NCBI | http://www.ncbi.nlm.nih.gov/geo/ | 数据库 | 基因表达谱 |
| Tefor | http://crispor.tefor.vet | 数据库 | CRISPOR引物在线设计 |
| PrimerX | http://www.bioinformatics.org/primerx/cgi-bin/DNA_1.cgi | 数据库 | 基因突变引物在线设计 |
| EDomics | http://edomics.qnlm.ac/#/home | 数据库 | 基因组和转录组等 |
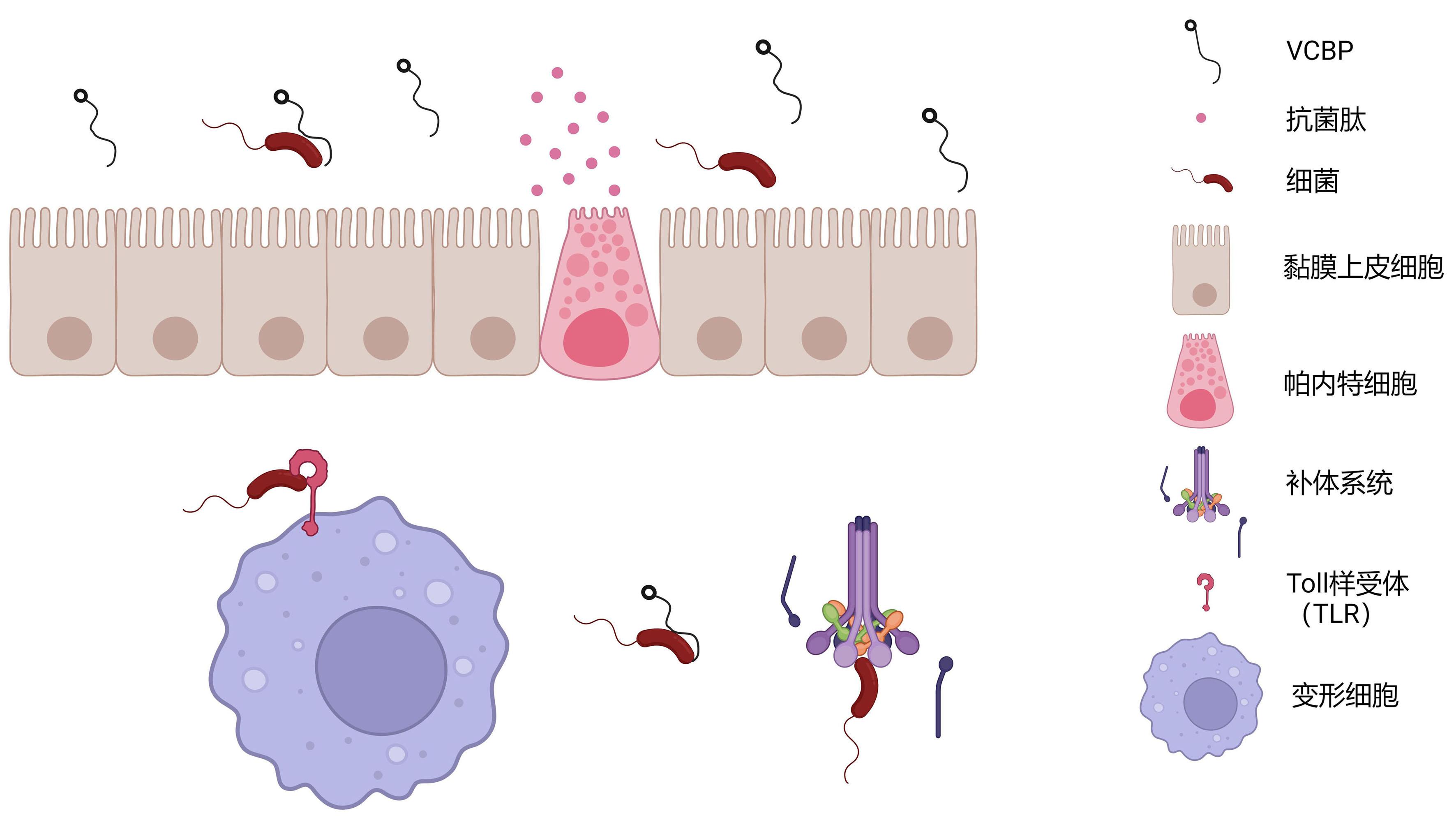
Figure 4 Protective mechanism of the innate immune system to the digestive tract of Ciona intestinalisNote: Mucosal immunity includes the secretion of mucus by mucosal epithelial cells and the secretion of antimicrobial peptides by Paneth cells. The innate immunity is mediated by Toll-like receptors (TLR), variable region-containing chitin-binding proteins (VCBP), and the complement system, which facilitate the phagocytic activity of amoebocytes.
免疫系统组成 Immune system composition | 玻璃海鞘 Ciona intestinalis | 人 Homo sapiens | 进化差别 Evolutionary difference | ||
|---|---|---|---|---|---|
分子 Molecules | 作用 Function | 分子 Molecules | 作用 Function | ||
免疫识别分子 Immune recognition molecules | TLR | 介导免疫应答 | TLR | 介导免疫应答 | 人类TLR具有更多亚型 |
| 凝集素 | 特异性结合糖蛋白 | - | - | 不存在同源 分子 | |
| 酚氧化酶 | 杀死细菌,合成黑色素 | - | - | 不存在同源 分子 | |
| CrPxt | 介导细胞-细胞,细胞-细胞外基质之间的黏附 | - | - | ||
| CrPtx-like | 参与体液免疫 | C反应蛋白 | 参与固有免疫反应 | 同源 | |
| 补体系统分子C3、CFB、MASP等 | 调理作用、炎症反应 | 补体系统分子C3、CFB、MASP等 | 调理作用、炎症反应、溶解细胞 | 同源 | |
细胞因子 Cytokine | TNF-α | 参与细菌的免疫反应 | TNF-α | 识别病原体,激活NF-κB | 同源 |
IL-17 | 介导抗真菌和细菌的保护性免疫 | IL-17 | 介导抗真菌和细菌的保护性免疫,也与多种自身免疫性和慢性炎症性疾病相关 | 同源 | |
| CiTGF-β | 参与免疫应答,激活免疫细胞 | hTGF-β | 调节炎症过程 | 同源 | |
组织重构与伤口愈合相关因子 Factors related to tissue remodeling and wound healing | MMP-9样分子 | 胚胎发生、组织重构、血管生成、伤口愈合 | MMP-9 | 胚胎发生、组织重构、血管生成、伤口愈合 | 同源 |
| MIF | 介导免疫反应 | MIF | 介导固有免疫和适应性免疫反应 | 同源 | |
顺式调控元件 Cis-regulatory element | Cr8long、Cr8short | 功能尚不明确 | RTP | 与嗅觉相关 | 同源 |
| CrCAP | 免疫应答调控 | GAIT复合物 | 免疫应答调控 | 同源 | |
抗菌肽 Antimicrobial peptide | Ci-MAM-A、 Ci-PAP-A | 参与初次免疫应答 | - | - | 不存在同源 分子 |
Table 2 Comparison of immune systems between Ciona intestinalis and human
免疫系统组成 Immune system composition | 玻璃海鞘 Ciona intestinalis | 人 Homo sapiens | 进化差别 Evolutionary difference | ||
|---|---|---|---|---|---|
分子 Molecules | 作用 Function | 分子 Molecules | 作用 Function | ||
免疫识别分子 Immune recognition molecules | TLR | 介导免疫应答 | TLR | 介导免疫应答 | 人类TLR具有更多亚型 |
| 凝集素 | 特异性结合糖蛋白 | - | - | 不存在同源 分子 | |
| 酚氧化酶 | 杀死细菌,合成黑色素 | - | - | 不存在同源 分子 | |
| CrPxt | 介导细胞-细胞,细胞-细胞外基质之间的黏附 | - | - | ||
| CrPtx-like | 参与体液免疫 | C反应蛋白 | 参与固有免疫反应 | 同源 | |
| 补体系统分子C3、CFB、MASP等 | 调理作用、炎症反应 | 补体系统分子C3、CFB、MASP等 | 调理作用、炎症反应、溶解细胞 | 同源 | |
细胞因子 Cytokine | TNF-α | 参与细菌的免疫反应 | TNF-α | 识别病原体,激活NF-κB | 同源 |
IL-17 | 介导抗真菌和细菌的保护性免疫 | IL-17 | 介导抗真菌和细菌的保护性免疫,也与多种自身免疫性和慢性炎症性疾病相关 | 同源 | |
| CiTGF-β | 参与免疫应答,激活免疫细胞 | hTGF-β | 调节炎症过程 | 同源 | |
组织重构与伤口愈合相关因子 Factors related to tissue remodeling and wound healing | MMP-9样分子 | 胚胎发生、组织重构、血管生成、伤口愈合 | MMP-9 | 胚胎发生、组织重构、血管生成、伤口愈合 | 同源 |
| MIF | 介导免疫反应 | MIF | 介导固有免疫和适应性免疫反应 | 同源 | |
顺式调控元件 Cis-regulatory element | Cr8long、Cr8short | 功能尚不明确 | RTP | 与嗅觉相关 | 同源 |
| CrCAP | 免疫应答调控 | GAIT复合物 | 免疫应答调控 | 同源 | |
抗菌肽 Antimicrobial peptide | Ci-MAM-A、 Ci-PAP-A | 参与初次免疫应答 | - | - | 不存在同源 分子 |
| 1 | DENOEUD F, HENRIET S, MUNGPAKDEE S, et al. Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate[J]. Science, 2010, 330( 6009): 1381- 1385. DOI: 10.1126/science.1194167 . |
| 2 | DELSUC F, BRINKMANN H, CHOURROUT D, et al. Tunicates and not cephalochordates are the closest living relatives of vertebrates[J]. Nature, 2006, 439( 7079): 965- 968. DOI: 10.1038/nature04336 . |
| 3 | TREEN N, SHIMOBAYASHI S F, EEFTENS J, et al. Properties of repression condensates in living Ciona embryos[J]. Nat Commun, 2021, 12( 1): 1561. DOI: 10.1038/s41467-021-21606-5 . |
| 4 | SATOU Y, NAKAMURA R, YU D L, et al. A nearly complete genome of Ciona intestinalis type A (C. robusta) reveals the contribution of inversion to chromosomal evolution in the genus Ciona [J]. Genome Biol Evol, 2019, 11( 11): 3144- 3157. DOI: 10.1093/gbe/evz228 . |
| 5 | DEHAL P, SATOU Y, CAMPBELL R K, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins[J]. Science, 2002, 298( 5601): 2157- 2167. DOI: 10.1126/science.1080049 . |
| 6 | SATOU Y, TOKUOKA M, ODA-ISHII I, et al. A manually curated gene model set for an ascidian, Ciona robusta ( Ciona intestinalis type a)[J]. Zoolog Sci, 2022, 39( 3): 253- 260. DOI: 10.2108/zs210102 . |
| 7 | SATOH N, SATOU Y, DAVIDSON B, et al. Ciona intestinalis: an emerging model for whole-genome analyses[J]. Trends Genet, 2003, 19( 7): 376- 381. DOI: 10.1016/S0168-9525(03)00144-6 . |
| 8 | LIBERTI A, NATARAJAN O, ATKINSON C G F, et al. Reflections on the use of an invertebrate chordate model system for studies of gut microbial immune interactions[J]. Front Immunol, 2021, 12: 642687. DOI: 10.3389/fimmu. 2021. 642687 . |
| 9 | MATSUBARA S, KAWADA T, SAKAI T, et al. The significance of Ciona intestinalis as a stem organism in integrative studies of functional evolution of the chordate endocrine, neuroendocrine, and nervous systems[J]. Gen Comp Endocrinol, 2016, 227: 101- 108. DOI: 10.1016/j.ygcen.2015.05.010 . |
| 10 | HAYWOOD C A, MOON H P, MILLAR R H. Reversal of the heart-beat in Tunicates[J]. Nature, 1953, 172( 4366): 40- 41. DOI: 10.1038/172040b0 . |
| 11 | KALK M. The organization of a tunicate heart[J]. Tissue Cell, 1970, 2( 1): 99- 118. DOI: 10.1016/s0040-8166(70)80010-6 . |
| 12 | EALES J G. Iodine metabolism and thyroid-related functions in organisms lacking thyroid follicles: are thyroid hormones also vitamins?[J]. Proc Soc Exp Biol Med, 1997, 214( 4): 302- 317. DOI: 10.3181/00379727-214-44098 . |
| 13 | PASSAMANECK Y J, DI GREGORIO A. Ciona intestinalis: chordate development made simple[J]. Dev Dyn, 2005, 233( 1): 1- 19. DOI: 10.1002/dvdy.20300 . |
| 14 | YOSHIDA K, TREEN N. TALEN-based knockout system[M]//SASAKURA Y, ed. Transgenic Ascidians. Singapore: Springer, 2018: 131- 139. DOI: 10.1007/978-981-10-7545-2_12 . |
| 15 | NAKASHIMA K, YAMADA L, SATOU Y, et al. The evolutionary origin of animal cellulose synthase[J]. Dev Genes Evol, 2004, 214( 2): 81- 88. DOI: 10.1007/s00427-003-0379-8 . |
| 16 | GANDHI S, RAZY-KRAJKA F, CHRISTIAEN L, et al. CRISPR knockouts in Ciona embryos[J]. Adv Exp Med Biol, 2018, 1029: 141- 152. DOI: 10.1007/978-981-10-7545-2_13 . |
| 17 | SASAKURA Y, HORIE T. Improved genome editing in the ascidian Ciona with CRISPR/Cas9 and TALEN[J]. Methods Mol Biol, 2023, 2637: 375- 388. DOI: 10.1007/978-1-0716-3016-7_28 . |
| 18 | SATOU Y, IMAI K S, SATOH N. Action of morpholinos in Ciona embryos[J]. Genesis, 2001, 30( 3): 103- 106. DOI: 10.1002/gene.1040 . |
| 19 | HOZUMI A, KAWAI N, YOSHIDA R, et al. Efficient transposition of a single Minos transposon copy in the genome of the ascidian Ciona intestinalis with a transgenic line expressing transposase in eggs[J]. Dev Dyn, 2010, 239( 4): 1076- 1088. DOI: 10.1002/dvdy.22254 . |
| 20 | KOBAYASHI K, SATOU Y. Microinjection of exogenous nucleic acids into eggs: Ciona species[M]//SASAKURA Y. Transgenic Ascidians. Singapore: Springer, 2018: 5- 13.10. 1007/978-981-10-7545-2_ 2. |
| 21 | ZELLER R W. Electroporation in ascidians: history, theory and protocols[M]//SASAKURA Y. Transgenic Ascidians. Singapore: Springer, 2018: 37- 48.10. 1007/978-981-10-7545-2_ 5. |
| 22 | HORIE T, NAKAGAWA M, SASAKURA Y, et al. Cell type and function of neurons in the ascidian nervous system[J]. Dev Growth Differ, 2009, 51( 3): 207- 220. DOI: 10.1111/j.1440-169X.2009.01105.x . |
| 23 | HORIE T, NAKAGAWA M, SASAKURA Y, et al. Simple motor system of the ascidian larva: neuronal complex comprising putative cholinergic and GABAergic/glycinergic neurons[J]. Zoolog Sci, 2010, 27( 2): 181- 190. DOI: 10.2108/zsj.27.181 . |
| 24 | STOLFI A, LEVINE M. Neuronal subtype specification in the spinal cord of a protovertebrate[J]. Development, 2011, 138( 5): 995- 1004. DOI: 10.1242/dev.061507 . |
| 25 | IKUTA T, SAIGA H. Dynamic change in the expression of developmental genes in the ascidian central nervous system: revisit to the tripartite model and the origin of the midbrain-hindbrain boundary region[J]. Dev Biol, 2007, 312( 2): 631- 643. DOI: 10.1016/j.ydbio.2007.10.005 . |
| 26 | IMAI K S, STOLFI A, LEVINE M, et al. Gene regulatory networks underlying the compartmentalization of the Ciona central nervous system[J]. Development, 2009, 136( 2): 285- 293. DOI: 10.1242/dev.026419 . |
| 27 | LEMAIRE L A, CAO C, YOON P H, et al. The hypothalamus predates the origin of vertebrates[J]. Sci Adv, 2021, 7( 18): eabf7452. DOI: 10.1126/sciadv.abf7452 . |
| 28 | OSUGI T, SASAKURA Y, SATAKE H. The nervous system of the adult ascidian Ciona intestinalis Type A ( Ciona robusta): insights from transgenic animal models[J]. PLoS One, 2017, 12( 6): e0180227. DOI: 10.1371/journal.pone.0180227 . |
| 29 | TSUDA M, KAWAKAMI I, SHIRAISHI S. Sensitization and habituation of the swimming behavior in ascidian larvae to light[J]. Zoolog Sci, 2003, 20( 1): 13- 22. DOI: 10.2108/zsj.20.13 . |
| 30 | BORBA C, KOURAKIS M J, SCHWENNICKE S, et al. Fold change detection in visual processing[J]. Front Neural Circuits, 2021, 15: 705161. DOI: 10.3389/fncir.2021.705161 . |
| 31 | SATAKE H, MATSUBARA S, SHIRAISHI A, et al. Neuropeptides, peptide hormones, and their receptors of a tunicate, Ciona intestinalis [J]. Results Probl Cell Differ, 2019, 68: 107- 125. DOI: 10.1007/978-3-030-23459-1_5 . |
| 32 | AOYAMA M, KAWADA T, FUJIE M, et al. A novel biological role of tachykinins as an up-regulator of oocyte growth: identification of an evolutionary origin of tachykininergic functions in the ovary of the ascidian, Ciona intestinalis [J]. Endocrinology, 2008, 149( 9): 4346- 4356. DOI: 10.1210/en.2008-0323 . |
| 33 | KAWADA T, OGASAWARA M, SEKIGUCHI T, et al. Peptidomic analysis of the central nervous system of the protochordate, Ciona intestinalis: homologs and prototypes of vertebrate peptides and novel peptides[J]. Endocrinology, 2011, 152( 6): 2416- 2427. DOI: 10.1210/en.2010-1348 . |
| 34 | HOZUMI A, MATSUNOBU S, MITA K R, et al. GABA-induced GnRH release triggers chordate metamorphosis[J]. Curr Biol, 2020, 30( 8): 1555- 1561.e4. DOI: 10.1016/j.cub.2020.02.003 . |
| 35 | AZUMI K, DE SANTIS R, DE TOMASO A, et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: "waiting for Godot"[J]. Immunogenetics, 2003, 55( 8): 570- 581. DOI: 10.1007/s00251-003-0606-5 . |
| 36 | SATAKE H, MATSUBARA S, SHIRAISHI A, et al. Peptide receptors and immune-related proteins expressed in the digestive system of a urochordate, Ciona intestinalis [J]. Cell Tissue Res, 2019, 377( 3): 293- 308. DOI: 10.1007/s00441-019-03024-8 . |
| 37 | LONGO V, PARRINELLO D, LONGO A, et al. The conservation and diversity of ascidian cells and molecules involved in the inflammatory reaction: the Ciona robusta model[J]. Fish Shellfish Immunol, 2021, 119: 384- 396. DOI: 10.1016/j.fsi.2021.10.022 . |
| 38 | SATOH N. Innate immune system and blood cells[M/OL]//SATOH N.Developmental genomics of ascidians.Hoboken:John Wiley & Sons,Inc., 2013:159-165.(2013-12-05)[2023-11-01].https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118656129.ch15. |
| 39 | DI BELLA M A, FEDDERS H, DE LEO G, et al. Localization of antimicrobial peptides in the tunic of Ciona intestinalis (Ascidiacea, Tunicata) and their involvement in local inflammatory-like reactions[J]. Results Immunol, 2011, 1( 1): 70- 75. DOI: 10.1016/j.rinim.2011.09.001 . |
| 40 | KASAHARA M, SUZUKI T, PASQUIER L D. On the origins of the adaptive immune system: novel insights from invertebrates and cold-blooded vertebrates[J]. Trends Immunol, 2004, 25( 2): 105- 111. DOI: 10.1016/j.it.2003.11.005 . |
| 41 | DISHAW L J, CANNON J P, LITMAN G W, et al. Immune-directed support of rich microbial communities in the gut has ancient roots[J]. Dev Comp Immunol, 2014, 47( 1): 36- 51. DOI: 10.1016/j.dci.2014.06.011 . |
| 42 | VIZZINI A, PARRINELLO D, SANFRATELLO M A, et al. Ciona intestinalis peroxinectin is a novel component of the peroxidase-cyclooxygenase gene superfamily upregulated by LPS[J]. Dev Comp Immunol, 2013, 41( 1): 59- 67. DOI: 10.1016/j.dci.2013.03.015 . |
| 43 | VIZZINI A, BONURA A, LONGO V, et al. Isolation of a novel LPS-induced component of the ML superfamily in Ciona intestinalis [J]. Dev Comp Immunol, 2015, 53( 1): 70- 78. DOI: 10.1016/j.dci.2015.06.018 . |
| 44 | VIZZINI A, BONURA A, LA PAGLIA L, et al. Transcriptomic analyses reveal 2 and 4 family members of cytochromes P450 (CYP) involved in LPS inflammatory response in pharynx of Ciona robusta [J]. Int J Mol Sci, 2021, 22( 20): 11141. DOI: 10.3390/ijms222011141 . |
| 45 | BONURA A, VIZZINI A, VLAH S, et al. Ci8 short, a novel LPS-induced peptide from the ascidian Ciona intestinalis, modulates responses of the human immune system[J]. Immunobiology, 2018, 223( 2): 210- 219. DOI: 10.1016/j.imbio. 2017.10.024 . |
| 46 | ARIZZA V, BONURA A, LA PAGLIA L, et al. Transcriptional and in silico analyses of MIF cytokine and TLR signalling interplay in the LPS inflammatory response of Ciona robusta [J]. Sci Rep, 2020, 10( 1): 11339. DOI: 10.1038/s41598-020-68339-x . |
| 47 | VIZZINI A, DUMAS F, DI FALCO F, et al. Evolutionary and transcriptional analyses of a pentraxin-like component family involved in the LPS inflammatory response of Ciona robusta [J]. Fish Shellfish Immunol, 2021, 111: 94- 101. DOI: 10.1016/j.fsi.2021.01.014 . |
| 48 | STEMPLE D L. Structure and function of the notochord: an essential organ for chordate development[J]. Development, 2005, 132( 11): 2503- 2512. DOI: 10.1242/dev.01812 . |
| 49 | LU Q X, BHATTACHAN P, DONG B. Ascidian notochord elongation[J]. Dev Biol, 2019, 448( 2): 147- 153. DOI: 10.1016/j.ydbio.2018.11.009 . |
| 50 | DONG B, HORIE T, DENKER E, et al. Tube formation by complex cellular processes in Ciona intestinalis notochord[J]. Dev Biol, 2009, 330( 2): 237- 249. DOI: 10.1016/j.ydbio.2009.03.015 . |
| 51 | MUNRO E M, ODELL G M. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord[J]. Development, 2002, 129( 1): 13- 24. DOI: 10.1242/dev.129.1.13 . |
| 52 | BAGNAT M, CHEUNG I D, MOSTOV K E, et al. Genetic control of single lumen formation in the zebrafish gut[J]. Nat Cell Biol, 2007, 9: 954- 960. DOI: 10.1038/ncb1621 . |
| 53 | WANG Z Q, OUYANG X K, TAN Z C, et al. Quantitative phosphoproteomics reveals the requirement of DYRK1-mediated phosphorylation of ion transport- and cell junction-related proteins for notochord lumenogenesis in ascidian[J]. Cells, 2023, 12( 6): 921. DOI: 10.3390/cells12060921 . |
| 54 | DI G A. The notochord gene regulatory network in chordate evolution: conservation and divergence from Ciona to vertebrates[J]. Curr Top Dev Biol, 2020, 139: 325- 374. DOI: 10.1016/bs.ctdb.2020.01.002 . |
| 55 | OLIVO P, PALLADINO A, RISTORATORE F, et al. Brain sensory organs of the ascidian Ciona robusta: structure, function and developmental mechanisms[J]. Front Cell Dev Biol, 2021, 9: 701779. DOI: 10.3389/fcell.2021.701779 . |
| 56 | ISLAS J F, LIU Y, WENG K C, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors[J]. Proc Natl Acad Sci USA, 2012, 109( 32): 13016- 13021. DOI: 10.1073/pnas.1120299109 . |
| 57 | WANG W, NIU X, STUART T, et al. A single-cell transcriptional roadmap for cardiopharyngeal fate diversification[J]. Nat Cell Biol, 2019, 21( 6): 674- 686. DOI: 10.1038/s41556-019-0336-z . |
| 58 | AUGER H, SASAKURA Y, JOLY J S, et al. Regeneration of oral siphon pigment organs in the ascidian Ciona intestinalis [J]. Dev Biol, 2010, 339( 2): 374- 389. DOI: 10.1016/j.ydbio.2009. 12.040 . |
| 59 | JEFFERY W R. Closing the wounds: one hundred and twenty five years of regenerative biology in the ascidian Ciona intestinalis [J]. Genesis, 2015, 53( 1): 48- 65. DOI: 10.1002/dvg. 22799 . |
| 60 | JEFFERY W R. Distal regeneration involves the age dependent activity of branchial sac stem cells in the ascidian Ciona intestinalis [J]. Regeneration, 2015, 2( 1): 1- 18. DOI: 10.1002/reg2.26 . |
| 61 | JEFFERY W R. Regeneration, stem cells, and aging in the tunicate Ciona: insights from the oral siphon[J]. Int Rev Cell Mol Biol, 2015, 319: 255- 282. DOI: 10.1016/bs.ircmb.2015.06.005 . |
| 62 | JEFFERY W R, GORIČKI Š. Apoptosis is a generator of Wnt-dependent regeneration and homeostatic cell renewal in the ascidian Ciona [J]. Biol Open, 2021, 10( 4): bio058526. DOI: 10.1242/bio.058526 . |
| 63 | JEFFERY W R, LI B, NG M, et al. Differentially expressed chaperone genes reveal a stress response required for unidirectional regeneration in the basal chordate Ciona [J]. BMC Biol, 2023, 21( 1): 148. DOI: 10.1186/s12915-023-01633-y . |
| 64 | BOLLNER T, BEESLEY P W, THORNDYKE M C. Pattern of substance P- and cholecystokinin-like immunoreactivity during regeneration of the neural complex in the ascidian Ciona intestinalis [J]. J Comp Neurol, 1992, 325( 4): 572- 580. DOI: 10.1002/cne.903250409 . |
| 65 | DAHLBERG C, AUGER H, DUPONT S, et al. Refining the Ciona intestinalis model of central nervous system regeneration[J]. PLoS One, 2009, 4( 2): e4458. DOI: 10.1371/journal.pone.0004458 . |
| 66 | JOLY J S. Aquatic model organisms in neurosciences: the genome-editing revolution[M]// Genome Editing in Neurosciences. Cham: Springer, 2017: 21- 29.10. 1007/978-3-319-60192-2_ 2. |
| 67 | HARDY J A, HIGGINS G A. Alzheimer's disease: the amyloid cascade hypothesis[J]. Science, 1992, 256( 5054): 184- 185. DOI: 10.1126/science.1566067 . |
| 68 | SCHNABEL J. Amyloid: little proteins, big clues[J]. Nature, 2011, 475( 7355): S12- S14. DOI: 10.1038/475S12a . |
| 69 | VIRATA M J, ZELLER R W. Ascidians: an invertebrate chordate model to study Alzheimer's disease pathogenesis[J]. Dis Model Mech, 2010, 3( 5-6): 377- 385. DOI: 10.1242/dmm.003434 . |
| 70 | GÖTZ J, STREFFER J R, DAVID D, et al. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy[J]. Mol Psychiatry, 2004, 9( 7): 664- 683. DOI: 10.1038/sj.mp.4001508 . |
| 71 | SCHUSTER K J, CHRISTIAEN L. The chordate origins of heart regeneration[J]. bioRxiv, 2023: 2023.09. 19. 558507. DOI: 10.1101/2023.09.19.558507 . |
| 72 | GARCIA J, CALLEWAERT N, BORSIG L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells[J]. Glycobiology, 2007, 17( 2): 185- 196. DOI: 10.1093/glycob/cwl059 . |
| 73 | OZAWA H, SONODA Y, KATO S, et al. Sulfatides inhibit adhesion, migration, and invasion of murine melanoma B16F10 cell line in vitro [J]. Biol Pharm Bull, 2012, 35( 11): 2054- 2058. DOI: 10.1248/bpb.b12-00492 . |
| 74 | CHENG L Y, LIU M, WANG C C, et al. CI431, an aqueous compound from Ciona intestinalis L., induces apoptosis through a mitochondria-mediated pathway in human hepatocellular carcinoma cells[J]. Evid Based Complement Alternat Med, 2011, 2011: 292873. DOI: 10.1155/2011/292873 . |
| 75 | FEDDERS H, MICHALEK M, GRÖTZINGER J, et al. An exceptional salt-tolerant antimicrobial peptide derived from a novel gene family of haemocytes of the marine invertebrate Ciona intestinalis [J]. Biochem J, 2008, 416( 1): 65- 75. DOI: 10.1042/BJ20080398 . |
| 76 | JENA P, MISHRA B, LEIPPE M, et al. Membrane-active antimicrobial peptides and human placental lysosomal extracts are highly active against mycobacteria[J]. Peptides, 2011, 32( 5): 881- 887. DOI: 10.1016/j.peptides.2011.03.002 . |
| 77 | CONSORTIUM T M. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse[J]. Nature, 2020, 583( 7817): 590- 595. DOI: 10.1038/s41586-020-2496-1 . |
| [1] | YANG Rong, ZHAO Yining, YANG Wenjing, ZHAO Shanming, CHAI Yujing, ZHANG Chengcai, YUAN Zheng, CUI Shufang. Effect of 60Co γ-ray Radiation on Spleen of Naked Mole Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 519-522. |
| [2] | CHEN Chao, CONG Wei, YANG Wen-jing, CUI Shu-fang. Comparative Study on Radiation Resistance of Naked Mole-rat and Mice [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 479-483. |
| [3] | ZHU Xiao-su,SONG yan,XU Shi-qmg . Application of Bombyx mori as Model Organism in Modern Biology [J]. Laboratory Animal and Comparative Medicine, 2009, 29(1): 61-65. |
| [4] | LI Jun, XU Shu-yun. Some Progress of Intact Animal Model in Antiinflammatory-immunomodulating Pharmacology [J]. Laboratory Animal and Comparative Medicine, 2002, 22(3): 162-167. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||