
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (1): 21-29.DOI: 10.12300/j.issn.1674-5817.2022.128
• Animal Model of Human Disease: Pharmacology • Previous Articles Next Articles
Ping YANG1, Li CUI1( )(
)( ), Cheng YU2, Zhiyue WEN1
), Cheng YU2, Zhiyue WEN1
Received:2022-08-17
Revised:2022-10-30
Online:2023-02-25
Published:2023-02-25
Contact:
Li CUI
CLC Number:
Ping YANG,Li CUI,Cheng YU,et al. Effects of Bevacizumab Injection on the Skin Wound Healing in Cynomolgus Monkeys[J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 21-29. DOI: 10.12300/j.issn.1674-5817.2022.128.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.128
| 项目 Item | 得分Score | 描述 Description |
|---|---|---|
伤口周围的皮肤颜色(距离伤口2 cm) Skin color around the wound (2 cm from the wound) | 0 | 正常颜色或粉红色 |
| 1 | 淡红色或有压之褪色的发红 | |
| 2 | 白色或灰白色 | |
| 3 | 深红色或压之不褪色的发红 | |
| 4 | 黑色或色素沉着过度 | |
伤口周围组织水肿 Edema in wound surrounding tissue | 0 | 无水肿或肿胀 |
| 1 | 伤口周围非凹陷水肿范围<2 cm | |
| 2 | 伤口周围非凹陷水肿范围≥2 cm | |
| 3 | 伤口周围凹陷水肿范围<2 cm | |
| 4 | 伤口周围凹陷水肿范围≥2 cm | |
渗出液类型 Type of exudate | 0 | 无渗出液 |
| 1 | 红色 | |
| 2 | 粉红色 | |
| 3 | 薄而透明,水性 | |
| 4 | 黄色或绿色,气味难闻 | |
渗出量 Exudate amount | 0 | 无渗出液 |
| 1 | 伤口组织微湿,但无法测量 | |
| 2 | 伤口组织湿润,浸泡<25%的敷料 | |
| 3 | 伤口组织湿润,浸泡敷料的25%~75% | |
| 4 | 伤口组织湿润,浸泡>75%的敷料 |
Table 1 The back wound score in cynomolgus monkeys
| 项目 Item | 得分Score | 描述 Description |
|---|---|---|
伤口周围的皮肤颜色(距离伤口2 cm) Skin color around the wound (2 cm from the wound) | 0 | 正常颜色或粉红色 |
| 1 | 淡红色或有压之褪色的发红 | |
| 2 | 白色或灰白色 | |
| 3 | 深红色或压之不褪色的发红 | |
| 4 | 黑色或色素沉着过度 | |
伤口周围组织水肿 Edema in wound surrounding tissue | 0 | 无水肿或肿胀 |
| 1 | 伤口周围非凹陷水肿范围<2 cm | |
| 2 | 伤口周围非凹陷水肿范围≥2 cm | |
| 3 | 伤口周围凹陷水肿范围<2 cm | |
| 4 | 伤口周围凹陷水肿范围≥2 cm | |
渗出液类型 Type of exudate | 0 | 无渗出液 |
| 1 | 红色 | |
| 2 | 粉红色 | |
| 3 | 薄而透明,水性 | |
| 4 | 黄色或绿色,气味难闻 | |
渗出量 Exudate amount | 0 | 无渗出液 |
| 1 | 伤口组织微湿,但无法测量 | |
| 2 | 伤口组织湿润,浸泡<25%的敷料 | |
| 3 | 伤口组织湿润,浸泡敷料的25%~75% | |
| 4 | 伤口组织湿润,浸泡>75%的敷料 |
指标及组别 Index and group | 手术后时间 Time after surgery/d | ||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | |
| WBC/(109·L-1) | |||||
| 生理盐水组 Saline group | 13.20±0.08 | 31.00±1.05△ | 14.65±0.10 | 15.64±0.08 | 18.70±0.69 |
| 贝伐珠单抗组 Bevacizumab group | 12.15±0.09 | 25.25±0.08△ | 13.48±0.04 | 15.72±0.50 | 17.14±0.23 |
| RBC/(1012·L-1) | |||||
| 生理盐水组 Saline group | 5.80±0.21 | 6.01±0.48 | 6.22±0.13 | 5.88±0.35 | 5.99±0.34 |
| 贝伐珠单抗组Bevacizumab group | 6.10±0.16 | 5.84±0.43 | 5.97±0.15 | 5.72±0.11 | 5.84±0.18 |
| NEU/% | |||||
| 生理盐水组 Saline group | 44.03±2.23 | 81.80±3.98△ | 50.97±4.83 | 61.57±6.75 | 60.73±17.91 |
| 贝伐珠单抗组 Bevacizumab group | 59.67±4.58 | 66.50±10.38 | 61.6±4.85 | 58.57±7.83 | 74.40±4.97 |
| NEU/(109·L-1) | |||||
| 生理盐水组 Saline group | 5.81±0.30 | 25.41±1.93△△ | 7.46±0.66 | 9.61±1.01 | 11.53±3.54 |
| 贝伐珠单抗组 Bevacizumab group | 7.26±0.59 | 16.80±2.62△ | 8.31±0.66 | 9.25±1.39 | 12.74±0.83 |
| LYM/% | |||||
| 生理盐水组 Saline group | 50.37±2.92 | 14.53±3.75 | 43.17±3.76 | 30.97±6.64 | 30.37±14.77 |
| 贝伐珠单抗组 Bevacizumab group | 33.30±4.35 | 28.43±9.75*△ | 33.93±5.04 | 34.53±7.23 | 20.30±4.66 |
| LYM/(109·L-1) | |||||
| 生理盐水组 Saline group | 6.65±0.38 | 4.44±1.08 | 6.33±0.59 | 4.86±1.06 | 5.52±2.52 |
| 贝伐珠单抗组 Bevacizumab group | 4.05±0.52 | 7.18±2.48 | 4.58±0.68 | 5.40±1.05 | 3.48±0.78 |
| HG1B ρ/(g·L-1) | |||||
| 生理盐水组 Saline group | 140.00±3.06 | 147.00±7.00 | 149.33±5.84 | 144.33±7.17 | 139.00±3.61 |
| 贝伐珠单抗组 Bevacizumab group | 150.00±5.57 | 142.33±7.06 | 142.67±7.84 | 140.67±2.73 | 140.33±3.84 |
| HCT/% | |||||
| 生理盐水组 Saline group | 45.93±1.13 | 47.27±2.20 | 47.93±2.15 | 45.73±2.22 | 44.97±1.90 |
| 贝伐珠单抗组 Bevacizumab group | 48.70±1.65 | 45.40±2.39 | 45.93±2.05 | 45.87±1.22 | 45.30±1.56 |
| EOS/% | |||||
| 生理盐水组 Saline group | 0.87±0.29 | 0.43±0.34 | 1.23±0.29 | 2.13±0.95 | 3.50±3.06 |
| 贝伐珠单抗组Bevacizumab group | 0.83±0.38 | 0.47±0.20 | 0.27±0.03 | 1.57±0.75 | 0.73±0.35 |
| EOS/(109·L-1) | |||||
| 生理盐水组 Saline group | 0.12±0.04 | 0.14±0.10 | 0.18±0.04 | 0.33±0.15 | 0.62±0.54 |
| 贝伐珠单抗组 Bevacizumab group | 0.10±0.05 | 0.11±0.05 | 0.03±0.01 | 0.24±0.11 | 0.13±0.06 |
Table 2 Blood routine examination results of cynomolgus monkeys
指标及组别 Index and group | 手术后时间 Time after surgery/d | ||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | |
| WBC/(109·L-1) | |||||
| 生理盐水组 Saline group | 13.20±0.08 | 31.00±1.05△ | 14.65±0.10 | 15.64±0.08 | 18.70±0.69 |
| 贝伐珠单抗组 Bevacizumab group | 12.15±0.09 | 25.25±0.08△ | 13.48±0.04 | 15.72±0.50 | 17.14±0.23 |
| RBC/(1012·L-1) | |||||
| 生理盐水组 Saline group | 5.80±0.21 | 6.01±0.48 | 6.22±0.13 | 5.88±0.35 | 5.99±0.34 |
| 贝伐珠单抗组Bevacizumab group | 6.10±0.16 | 5.84±0.43 | 5.97±0.15 | 5.72±0.11 | 5.84±0.18 |
| NEU/% | |||||
| 生理盐水组 Saline group | 44.03±2.23 | 81.80±3.98△ | 50.97±4.83 | 61.57±6.75 | 60.73±17.91 |
| 贝伐珠单抗组 Bevacizumab group | 59.67±4.58 | 66.50±10.38 | 61.6±4.85 | 58.57±7.83 | 74.40±4.97 |
| NEU/(109·L-1) | |||||
| 生理盐水组 Saline group | 5.81±0.30 | 25.41±1.93△△ | 7.46±0.66 | 9.61±1.01 | 11.53±3.54 |
| 贝伐珠单抗组 Bevacizumab group | 7.26±0.59 | 16.80±2.62△ | 8.31±0.66 | 9.25±1.39 | 12.74±0.83 |
| LYM/% | |||||
| 生理盐水组 Saline group | 50.37±2.92 | 14.53±3.75 | 43.17±3.76 | 30.97±6.64 | 30.37±14.77 |
| 贝伐珠单抗组 Bevacizumab group | 33.30±4.35 | 28.43±9.75*△ | 33.93±5.04 | 34.53±7.23 | 20.30±4.66 |
| LYM/(109·L-1) | |||||
| 生理盐水组 Saline group | 6.65±0.38 | 4.44±1.08 | 6.33±0.59 | 4.86±1.06 | 5.52±2.52 |
| 贝伐珠单抗组 Bevacizumab group | 4.05±0.52 | 7.18±2.48 | 4.58±0.68 | 5.40±1.05 | 3.48±0.78 |
| HG1B ρ/(g·L-1) | |||||
| 生理盐水组 Saline group | 140.00±3.06 | 147.00±7.00 | 149.33±5.84 | 144.33±7.17 | 139.00±3.61 |
| 贝伐珠单抗组 Bevacizumab group | 150.00±5.57 | 142.33±7.06 | 142.67±7.84 | 140.67±2.73 | 140.33±3.84 |
| HCT/% | |||||
| 生理盐水组 Saline group | 45.93±1.13 | 47.27±2.20 | 47.93±2.15 | 45.73±2.22 | 44.97±1.90 |
| 贝伐珠单抗组 Bevacizumab group | 48.70±1.65 | 45.40±2.39 | 45.93±2.05 | 45.87±1.22 | 45.30±1.56 |
| EOS/% | |||||
| 生理盐水组 Saline group | 0.87±0.29 | 0.43±0.34 | 1.23±0.29 | 2.13±0.95 | 3.50±3.06 |
| 贝伐珠单抗组Bevacizumab group | 0.83±0.38 | 0.47±0.20 | 0.27±0.03 | 1.57±0.75 | 0.73±0.35 |
| EOS/(109·L-1) | |||||
| 生理盐水组 Saline group | 0.12±0.04 | 0.14±0.10 | 0.18±0.04 | 0.33±0.15 | 0.62±0.54 |
| 贝伐珠单抗组 Bevacizumab group | 0.10±0.05 | 0.11±0.05 | 0.03±0.01 | 0.24±0.11 | 0.13±0.06 |
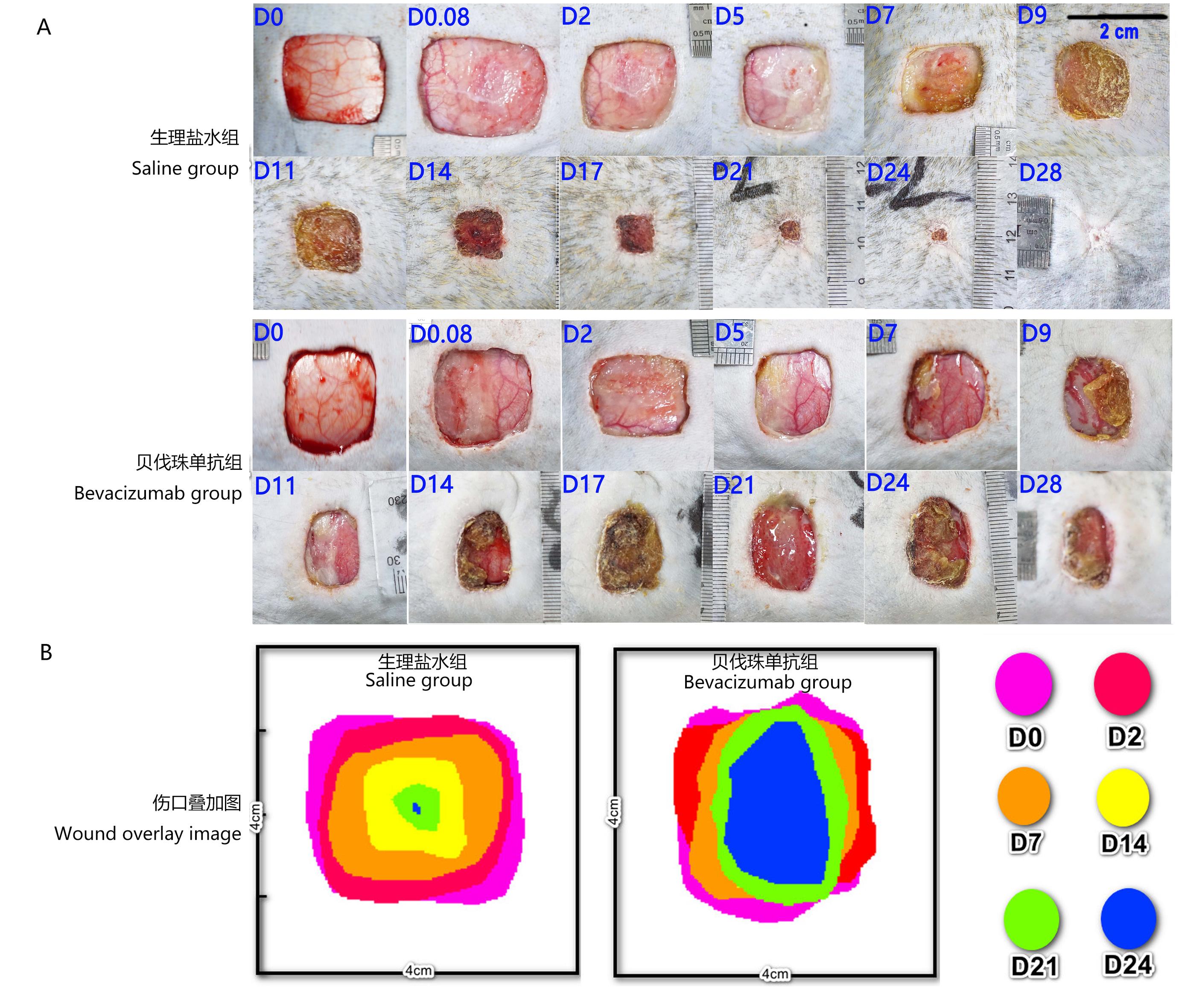
Figure 1 The picture of back wound state in cynomolgus monkeysNote:A, Representative wound pictures in saline group and Bevacizumab group, the resection area of skin was 4 cm×4 cm; B, Wound healing overlap diagrams in saline group and Bevacizumab group, which the 4 cm×4 cm images of the wound center were obtained at each superimposed time point. D0, D0.08, D2, D5, D7, D9, D11, D14, D17, D21, D24, and D28 represent minute 2, hour 2, day 2, day 5, day 7, day 9, day 11, day 14, day 17, day 21, day 24 and day 28 after full-thickness skin resection, respectively.
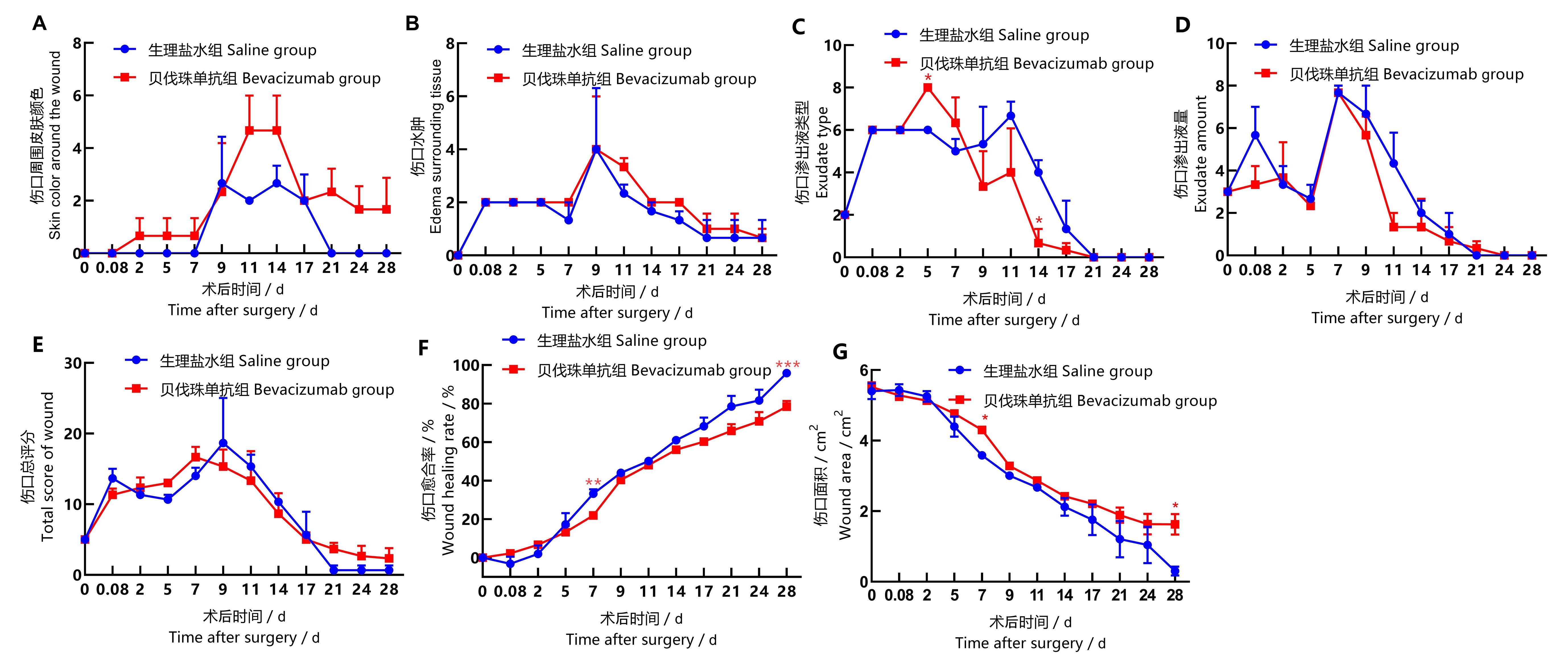
Figure 2 Statistical chart of back wound score and wound healing index in back of cynomolgus monkeysNote:T-test was used to compare Bevacizumab group with saline group at the same time point, *P<0.05, **P<0.01, ***P<0.001, n=3.
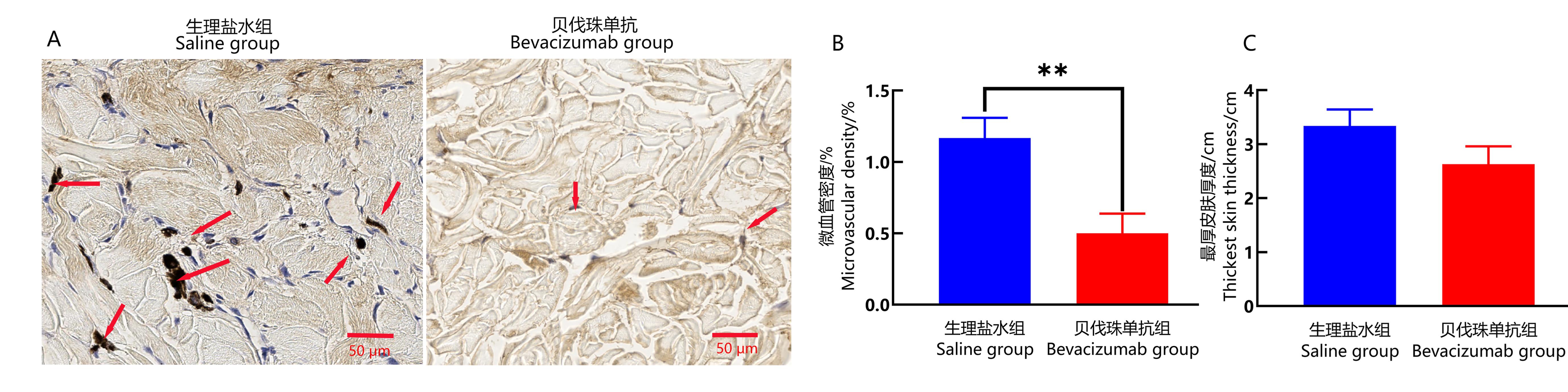
Figure 3 Immunohistochemical staining of CD34 and skin thickness analysis in the wound healing site of cynomolgus monkeysNote:A, CD34 immunohistochemical staining in the wound skin tissues of saline group and Bevacizumab group, showing skin characteristics of each group under 400× microscope with a scale bar of 50 μm. The arrow in the figure indicates that the CD34 in the cytoplasm of microvascular endothelial cells is stained with tan or brown particles.B, Comparison of percentage of microvessel area of CD34 positive expression (showing microvessel density) in the wound healing skin tissues between the two groups (**P<0.01, n=3). C, The thickness of the thickest part of newborn skin in the two groups.
| 1 | GUAN Z Z, XU J M, LUO R C, et al. Efficacy and safety of Bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phaseⅢ ARTIST trial[J]. Chin J Cancer, 2011, 30(10):682-689. DOI:10.5732/cjc.011.10188 . |
| 2 | NARITA Y. Bevacizumab for glioblastoma[J]. Ther Clin Risk Manag, 2015:1759. DOI:10.2147/tcrm.s58289 . |
| 3 | MAK D Y, TJONG M C, LOUIE A V. Usage of radiotherapy with osimertinib plus Bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC Harboring EGFR mutations[J]. J Thoracic Oncol, 2023, 18(1): e3-e4. DOI:https://doi.org/10.1016/j.jtho.2022.10.010 . |
| 4 | 寿建忠, 马建辉. 肾癌的靶向治疗现状与进展[J]. 中国新药杂志, 2010, 19(17):1539-1546. |
| SHOU J Z, MA J H. Current status and advances in targeted therapy for renal cell carcinoma[J]. Chin J New Drugs, 2010, 19(17):1539-1546. | |
| 5 | 唐欣颖, 匡泽民. «接受贝伐珠单抗治疗的卵巢癌和宫颈癌患者血压管理专家共识»: 2019英国专家建议解读[J]. 中国合理用药探索, 2020, 17(1):11-15. |
| TANG X Y, KUANG Z M. Interpretation of 2019 UK expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving Bevacizumab[J]. Chin J Ration Drug Use, 2020, 17(1):11-15. | |
| 6 | ZHANG H, HUANG Z, ZOU X, et al. Bevacizumab and wound-healing complications: A systematic review and meta-analysis of randomized controlled trials[J]. Oncotarget, 2016, 7(50): 82473-82481. DOI: 10.18632/oncotarget.12666 . |
| 7 | PRZEKORA A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro?[J]. Cells, 2020, 9(7):1622. DOI:10.3390/cells9071622 . |
| 8 | LAURENS N, KOOLWIJK P, DE MAAT M P M. Fibrin structure and wound healing[J]. J Thromb Haemost, 2006, 4(5):932-939. DOI:10.1111/j.1538-7836.2006.01861.x . |
| 9 | DE OLIVEIRA R C, WILSON S E. Fibrocytes, wound healing, and corneal fibrosis[J]. Invest Ophthalmol Vis Sci, 2020, 61(2):28. DOI:10.1167/iovs.61.2.28 . |
| 10 | URCIUOLO F, CASALE C, IMPARATO G, et al. Bioengineered skin substitutes: the role of extracellular matrix and vascularization in the healing of deep wounds[J]. J Clin Med, 2019, 8(12): E2083. DOI:10.3390/jcm8122083 . |
| 11 | VIG K, CHAUDHARI A, TRIPATHI S, et al. Advances in skin regeneration using tissue engineering[J]. Int J Mol Sci, 2017, 18(4): E789. DOI:10.3390/ijms18040789 . |
| 12 | HUGHES M R, CANALS HERNAEZ D, CAIT J, et al. A sticky wicket: defining molecular functions for CD34 in hematopoietic cells[J]. Exp Hematol, 2020, 86:1-14. DOI:10.1016/j.exphem.2020.05.004 . |
| 13 | KORNTNER S, LEHNER C, GEHWOLF R, et al. Limiting angiogenesis to modulate scar formation[J]. Adv Drug Deliv Rev, 2019, 146:170-189. DOI:10.1016/j.addr.2018.02.010 . |
| 14 | SAMI D G, HEIBA H H, ABDELLATIF A. Wound healing models: a systematic review of animal and non-animal models[J]. Wound Med, 2019, 24(1):8-17. DOI: 10.1016/j.wndm.2018.12.001 . |
| 15 | BYUN H S, LEE S J, LEE J I, et al. Effects of chitosan on wound healing in monkeys[J]. J Vet Clin, 2013, 30(4):241-246. |
| 16 | 中国药品评审中心. GPT1-1: 治疗用生物制品非临床安全性技术审评一般原则 [S]. 2007. |
| China Drug Review Center, GPT1-1: Guidelines for the non-clinical safety evaluation of therapeutic biological products [S]. 2007. | |
| 17 | 李劲锋, 于佳玥, 戴小宇, 等. 贝伐珠单抗类似药SMMU-13对食蟹猴的毒性[J]. 上海交通大学学报(农业科学版), 2018, 36(1):76-80. DOI:10.3969/J.ISSN.1671-9964.2018.01.013 . |
| LI J F, YU J Y, DAI X Y, et al. Toxicitystudy of SMMU-13, a biosimilar of Bevacizumab, in cynomolgus monkeys[J]. J Shanghai Jiao Tong Univ Agric Sci, 2018, 36(1):76-80. DOI:10.3969/J.ISSN.1671-9964.2018.01.013 . | |
| 18 | JIA Y, ZHAO G, JIA J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing[J]. J Ethnopharmacol, 2008, 120(2):181-189. DOI: 10.1016/j.jep.2008.08.008 . |
| 19 | APTE R S, CHEN D S, FERRARA N. VEGF in signaling and disease: Beyond discovery and development [J]. Cell, 2019, 176(6): 1248-1264. DOI:10.1016/j.cell.2019.01.021 . |
| 20 | GOLAS A R, BOYKO T, SCHWARTZ T H, et al. Prophylactic plastic surgery closure of neurosurgical scalp incisions reduces the incidence of wound complications in previously-operated patients treated with Bevacizumab (Avastin®) and radiation[J]. J Neurooncol, 2014, 119(2):327-331. DOI: 10.1007/s11060-014-1482-6 . |
| 21 | RIOS J E S, ALMEIDA F M, LIMONGI R M, et al. The effect of Bevacizumab, 5-fluorouracil, and triamcinolone on the healing modulation of surgical wounds in rats[J]. Histol Histopathol, 2023: 18583. DOI:10.14670/HH-18-583 . |
| 22 | ALMADANI Y H, VORSTENBOSCH J, DAVISON P G, et al. Wound healing: A comprehensive review[J]. Seminars in plastic surgery,2021, 35(3):141-144. DOI:10.1055/s-0041-1731791 . |
| 23 | 黄丽霞, 刘基华, 刘雪莹, 等. 贝伐珠单抗生物类似药与原研药疗效及不良反应的回顾性分析比较[J]. 中国处方药, 2022, 20(8):1-4. DOI: 10.3969/j.issn.1671-945X.2022.08.002 . |
| HUANG L X, LIU J H, LIU X Y, et al. Comparison of efficacy and adverse reactions between Bevacizumab biosimilar and the reference listed drug[J]. J China Prescr Drug, 2022, 20(8):1-4. DOI: 10.3969/j.issn.1671-945X.2022.08.002 . | |
| 24 | RECK M, WEHLER T, ORLANDI F, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without Bevacizumab versus Bevacizumab plus chemotherapy in non-small-cell lung cancer[J]. J Clin Oncol, 2020, 38(22):2530-2542. DOI:10.1200/jco.19.03158 . |
| 25 | FINN R S, QIN S, IKEDA M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20):1894-1905. DOI:10.1056/nejmoa1915745 . |
| 26 | AHN J W, SHALABI D, CORREA-SELM L M, et al. Impaired wound healing secondary to Bevacizumab[J]. Int Wound J, 2019, 16(4):1009-1012. DOI:10.1111/iwj.13139 . |
| 27 | 冯芬, 胡斌, 招丽蓉, 等. 血清VEGF水平与贝伐珠单抗联合化疗治疗转移性结直肠癌患者疗效的关系研究[J]. 现代生物医学进展, 2017, 17(31):6136-6139. DOI:10.13241/j.cnki.pmb.2017.31.032.FENG F , |
| HU B, ZHAO L R, et al. Metastasis colorectal cancer: effects of serum VEGF and bevacizumab combined with chemotherapy[J]. Prog Mod Biomed, 2017, 17(31):6136-6139. DOI: 10.13241/j.cnki.pmb.2017.31.032 . | |
| 28 | 张万虎, 何亚茹, 宋瑜, 等. 康柏西普与贝伐单抗玻璃体腔注射治疗新生血管性老年性黄斑变性疗效对比观察[J]. 当代医学, 2018, 24(8):12-15. DOI:10.3969/j.issn.1009-4393.2018.08.004 . |
| ZHANG W H, HE Y R, SONG Y, et al. Efficacy of conbercept versus Bevacizumab in intramuscular injection for neovascular age-related macular degeneration[J]. Contemp Med, 2018, 24(8):12-15. DOI: 10.3969/j.issn.1009-4393.2018.08.004 . | |
| 29 | SIMONDS J, MILLER F, MANDEL J, et al. The effect of Bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia[J]. Laryngoscope, 2009, 119(5):988-992. DOI:10.1002/lary.20159 . |
| 30 | KWAK D H, BAE T H, KIM W S, et al. Anti-vascular endothelial growth factor (Bevacizumab) therapy reduces hypertrophic scar formation in a rabbit ear wounding model[J]. Arch Plast Surg, 2016, 43(6):491-497. DOI:10.5999/aps.2016.43.6.491 . |
| [1] | WANG Chenjuan, YANG Lingyan, WANG Lipeng, SUN Xueping, LI Jingwen, GUO Lianxiang, RONG Rong, SHI Changjun. Diagnosis of an Outbreak of Canine Distemper in Cynomolgus Monkeys in an Experimental Monkey Farm in 2019 [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 360-367. |
| [2] | WEI Yanye, SHEN Guo, ZHANG Pengfei, SHI Songping, HU Jiahao, ZHANG Xuzhe, HUA Huiyuan, HUA Guanyang, LU Hongzheng, ZENG Yong, JI Feng, WEI Zhumei. Dynamic Monitoring and Correlation Analysis of General Body Indicators, Blood Glucose, and Blood Lipid in Obese Cynomolgus Monkeys [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 30-36. |
| [3] | Zhumei WEI, Guo SHEN, Zhenming LI, Yong ZENG, Feng JI, Jihong YANG. Measurement and Analysis of Bone Mineral Content and Bone Mineral Density in Healthy Cynomolgus Monkeys at Different Ages [J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 409-415. |
| [4] | GAO Shiping, LI Feng, ZHA Sifan. High-fat Diet Induced Cynomolgus Monkey Model of Non-alcoholic Fatty Liver Disease [J]. Laboratory Animal and Comparative Medicine, 2020, 40(2): 123-127. |
| [5] | LI Liang-shun, PAN Shi-you, ZHI Yuan-fang, BAI shuai-zhou, SUN Jian-hua, GONG Li-kun, REN Jin. Diagnosis and Treatment for Intussusceptions in One Case of Cynomolgus Monkey [J]. Laboratory Animal and Comparative Medicine, 2019, 39(3): 236-238. |
| [6] | WEI Zhu-mei, YANG Bo, LI Zhen-ming, HUAN Yin-hua, SU Ke-long, YANG Ji-hong. Continuous Monitoring on Blood Glucose and Insulin Levels in Obesity and Diabetes Cynomolgus Monkeys [J]. Laboratory Animal and Comparative Medicine, 2015, 35(5): 394-397. |
| [7] | YANG Bo, WEI Zhu-mei, DENG Xin-ning, HE Nai-zhi, YIN Feng-ri, LI Zhen-ming, YANG Ji-hong. Established Method of Measurement for Gastrocnemius Volume in Cynomolgus Monkeys by MRI Detection [J]. Laboratory Animal and Comparative Medicine, 2014, 34(4): 314-318. |
| [8] | SANG Chuan-lan, DUO Hai-gang, ZHANG Yong-Bin, CHEN Jia, Rao Jun-hua. Preliminary Probe of Detection of Simian Varicella Virus Antibodies in Cynomolgus Monkey [J]. Laboratory Animal and Comparative Medicine, 2012, 32(5): 446-448. |
| [9] | SHI Qiao-juan, LI wei, SA Xiao-ying. Application of Cynomolgus Monkey in Medical Science [J]. Laboratory Animal and Comparative Medicine, 2012, 32(4): 358-365. |
| [10] | ZENG Xian-cheng, JI Long-feng, ZHANG Ling-li, YAN Dong-ming, ZHOU Chao, JIANG Zi-rui, PAN Xue-ying, CHANG Yan, MA Jing. Determination of Cortisol in Plasma of Cynomolgus Monkeys by LC-MS/MS and Stress Monitoring [J]. Laboratory Animal and Comparative Medicine, 2011, 31(6): 441-446. |
| [11] | ZHAO Lei1,WANG Li1,JIANG Xu-cheng1,ZHEN Lin1,ZHANG Fan1,ZHAO Han-fang2,XU Wei-rong2. Effects of hVEGF Genetically Engineering Membrane on Expression of Fn and Integrin α5 During Healing Process of Full-thickness Cutaneous Defect of Rats [J]. Laboratory Animal and Comparative Medicine, 2009, 29(2): 100-104. |
| [12] | MA Nan-hua1,LI Zong-qiang2,QIN Jian1,ZHENG Li-ping1,ZHU Xu1,HUANG Sheng-xian1,XIE Li-ping3. Changes of Serum Levels of Estrogen and Progestogen during Menstrual Cycle in Normal Female Cynomolgus Monkeys [J]. Laboratory Animal and Comparative Medicine, 2008, 28(2): 106-109. |
| [13] | ZHAO Lei-1, WANG Li-1, JIANG Xu-Cheng-1, ZHENG Lin-1, ZHANG Fan-1, ZHAO Han-Fang-2, XU Wei-Rong-2. Model Establishing of Rat Skin Wounds Covered with hVEGF Genetically Engineering Membrane [J]. Laboratory Animal and Comparative Medicine, 2006, 26(1): 19-22. |
| [14] | WU Ji-Hong-1, ZHAO Yan-Fei-1, ZHENG Lin-1, ZHU De-An-2. The Role and Change of Mast Cells in Scalding Wound Healing in Rat [J]. Laboratory Animal and Comparative Medicine, 2000, 20(4): 198-201. |
| [15] | WANG Li-1, YAN Zhong-Fu-1, FENG Shi-Jie-2, FANG Pei-Yao-2, GUO Shou-Yan-1, WANG Bao-Mei-1. Pathological Observation on Wound Healing of Deep Partial Thickness Burn in Rats by Scalding with Different Length of Time [J]. Laboratory Animal and Comparative Medicine, 2000, 20(2): 79-81. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||